
EzriCare Recall Notice
Under the guidance and knowledge of the FDA and CDC, EzriCare Artificial Tears products are being recalled by Global Pharma Ltd.
The FDA and the CDC are warning that these products may cause serious injury, blindness, and possibly death due to a rare, serious bacterial infection.
EzriCare has done and is doing everything we can to protect the public, by warning everyone to immediately stop using these eyedrops and disposing of them safely to prevent accidental infection.
If you use these products and have experienced symptoms including eye pain, headache, or vision problems, please seek immediate medical attention.
If you have further questions about EzriCare Artifical Tears, please contact the recall hotline at 516-715-5181 for more information.
From the FDA recall website:
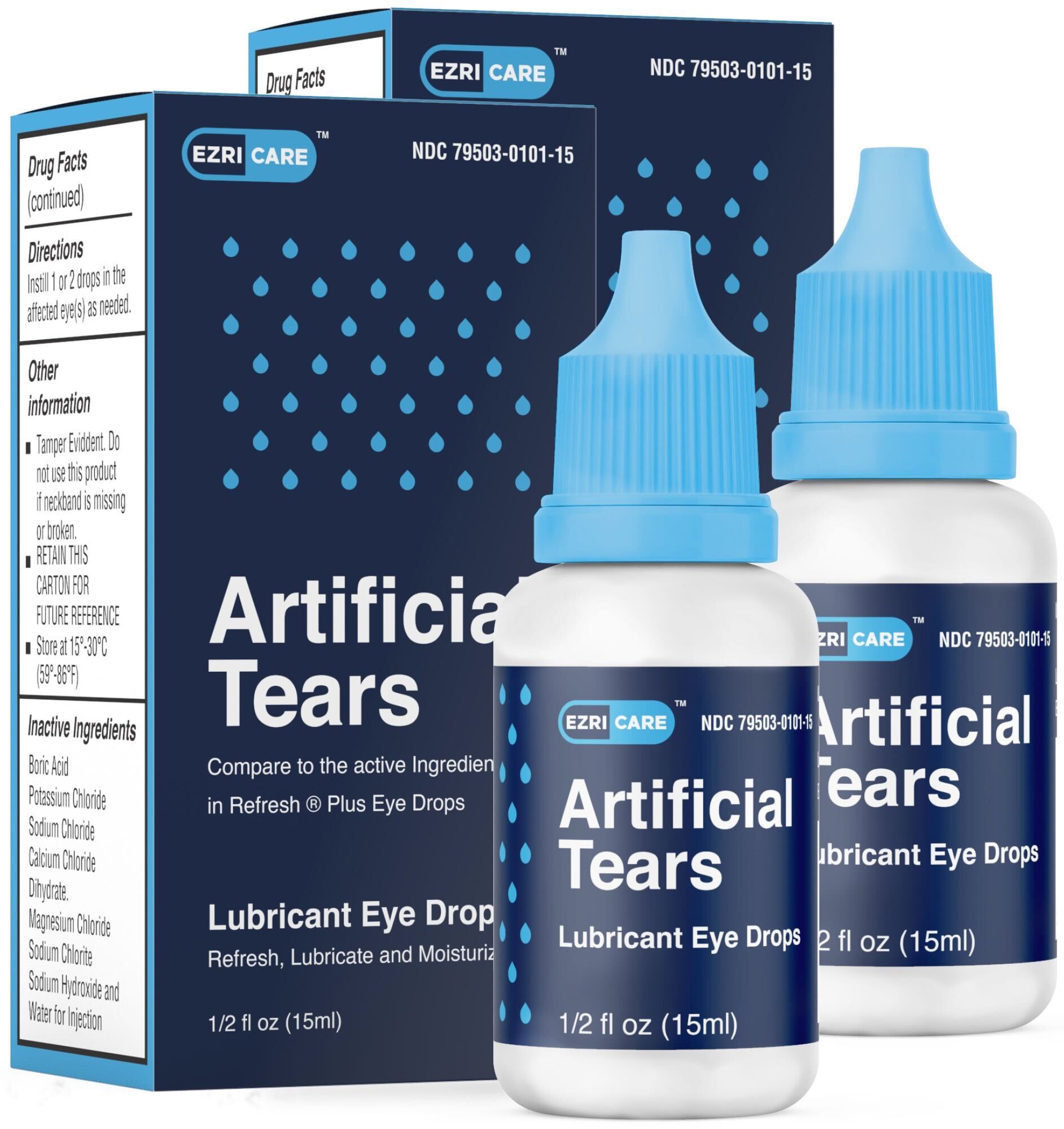
Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml) bottle are used as a protectant against further irritation or to relieve dryness of the eye for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun.
The product is packaged in a bottle with a safety seal and are placed in a carton box EzriCare NDC 79503-0101-15, UPC 3 79503 10115 7. It can be identified by the photos below. The product was distributed Nationwide in the USA over the Internet.

FDA encourages health care professionals and patients to report adverse events or quality problems with any medicine to FDA's MedWatch Adverse Event Reporting program:
Complete and submit the report online at Medwatch or Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Consumers may also report adverse reactions by contacting FDA's Consumer Complaint Coordinators.